-
Products
- Gas analysis systems
- GAOS SENSON gas analyzers
- GAOS MS process mass spectrometry
- MaOS HiSpec ion mobility spectrometer
- MaOS AxiSpec ion mobility spectrometer
- Applications
- News
- Events
- About us
In order to better understand the walls of plant cells, several microscopic and chemical methods have been used in recent years. However, there is a gap in knowledge regarding the location, number, and structural arrangement of molecules at the micro-level in the native cell wall. The advances in confocal Raman microscopy and the imaging approach solved this problem in a non-invasive way and provide chemical and structural information in-situ with a high spatial resolution (<0.5 µm).
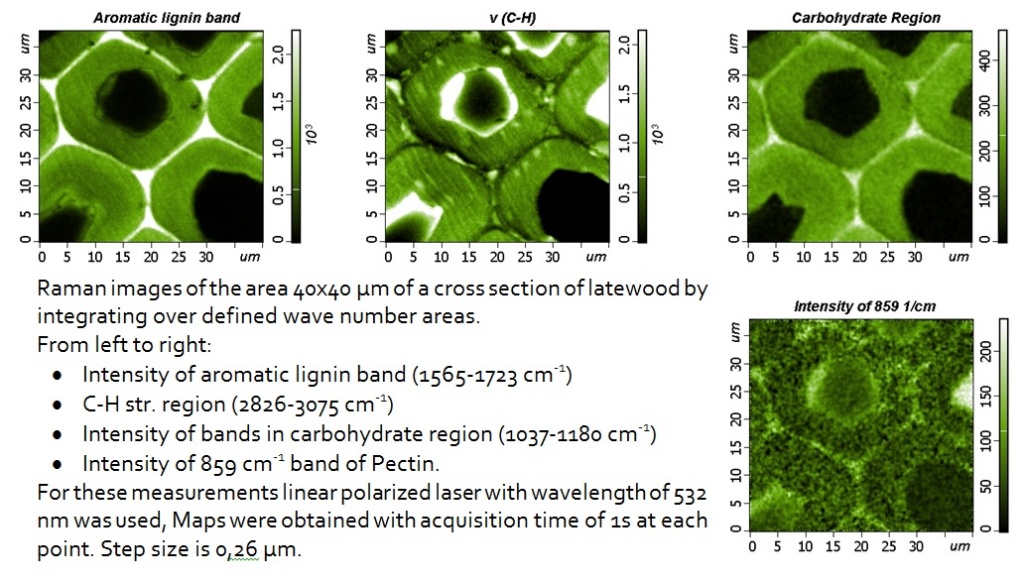
Examples of high-resolution Raman images are shown on wood cells, showing that changes in the chemical composition and orientation of polymers can be observed inside and between different layers of cell walls. Chemical images are based on changes in the integrated intensity or width of the Raman scattering bands due to the specific polymers of the plant cell wall and cellulose orientation.
Plant cell walls are complex and dynamic structures that determine the shape of plant and cell. Created from photosynthetically fixed carbon, they represent the most common source of terrestrial biomass and renewable energy. In addition, they are of practical importance for human nutrition and animals, as well as a source of natural fibers for textiles, materials and paper products. Thus, understanding the walls of plant cells is of great interest for many practical applications, both from a scientific and biomimetic point of view, since nature has created a large pool of walls of plant cells, differing in properties in accordance with the desired needs. In a broad sense, two types of cell walls can be distinguished: the primary walls are deposited during cell growth and the secondary cell walls after cessation of cell growth. Primary cell walls should be both mechanically stable and sufficiently tensile to allow cell expansion, avoiding cell rupture under their turgid pressure, and consist mainly of polysaccharides, which can be widely classified as cellulose, hemicellulose, and pectin. The last two classes of cell wall components are often called matrix polysaccharides. Secondary cell walls are deposited after cessation of cell growth and impart mechanical stability to specialized cell types, such as xylem elements and sclerenchyma cells. These walls are composites of cellulose and hemicellulose and are often saturated with lignins, an aromatic system consisting of phenylpropane units.
Cellulose crystals form a microfibrillar base in all plant cell walls. Although native cellulose, called cellulose I, was one of the most studied subjects in the science of polymers, the biosynthesis and ultrastructure of cellulose has not yet been fully resolved. The deceptive simplicity of the repeating binder of the β-1-4 cellobiose link does not indicate a complex arrangement of amorphous and crystalline regions at the level of fibrils and fibers. Inside the cell wall of plants, cellulose is embedded in matrix polymers, which play an important role in the development of the cell wall, as well as for its final properties. Polysaccharide matrix polymers, hemicelluloses, and pectins contain many different chemical configurations, as well as lignin, a polyphenolic inlaying polymer in the cell walls of secondary plants.
In addition, the secondary plant cell wall of the wood consists of different sublayers formed during different periods during cell differentiation. Between adjacent cells, the middle layer of the plate attached to the primary cell wall ensures the adhesion of the cell to its neighbors. The chemical composition of the cell wall and the alignment of cellulose microfibrils in different sublayers show significant interspecific and intraspecific variability and determine various properties of the cell wall.
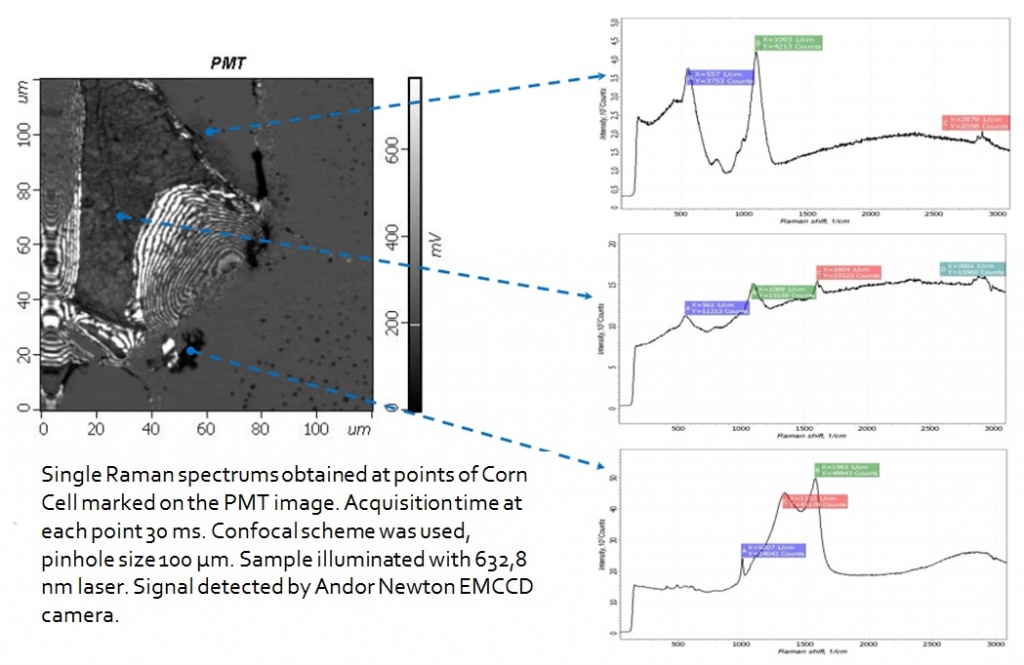
100x100 µm stage with capacitance sensors was used during the measurements.
Andor CCD Camera was used to detect a signal. Z scale represents detector counts.
Most traditional chemical analyzes of plant cell walls are destructive, as they require the destruction of plant tissue. But the functional characteristics of cell walls depend not only on the composition of the polymers of the cell wall but also on the fine details of their macromolecular structure and conformation and on their highly ordered architecture on a scale from a few nanometers to several microns. Most of these small details are lost when the cell wall polymers are fractionated and/or solubilized for study by classical chemical methods. In addition, difficulties with the selection of the sample arise when small areas of the cell wall or individual layers are of interest, as they must be carefully cut.
The combination of Raman and IR spectroscopy with microscopy has become an important toolkit in studies of the plant cell wall since they provide information about the molecular structure and composition in their natural state. Raman microspectroscopy has the advantage that the spectra can be obtained on aqueous, thicker, but not opaque samples with a higher spatial resolution. But the impedance of the autofluorescence of the sample, which can interfere with the analysis or limit the quality of the spectra, limited the application to plant tissues until the development of the method of NIR Raman scattering. For mapping and visualization of whole plant organs (seeds, fruits, leaves) the lateral resolution (10 μm) of the NIR method is adequate, whereas, for studies at a lower hierarchical level of cells and cell walls, high resolution is achieved using a visible laser.
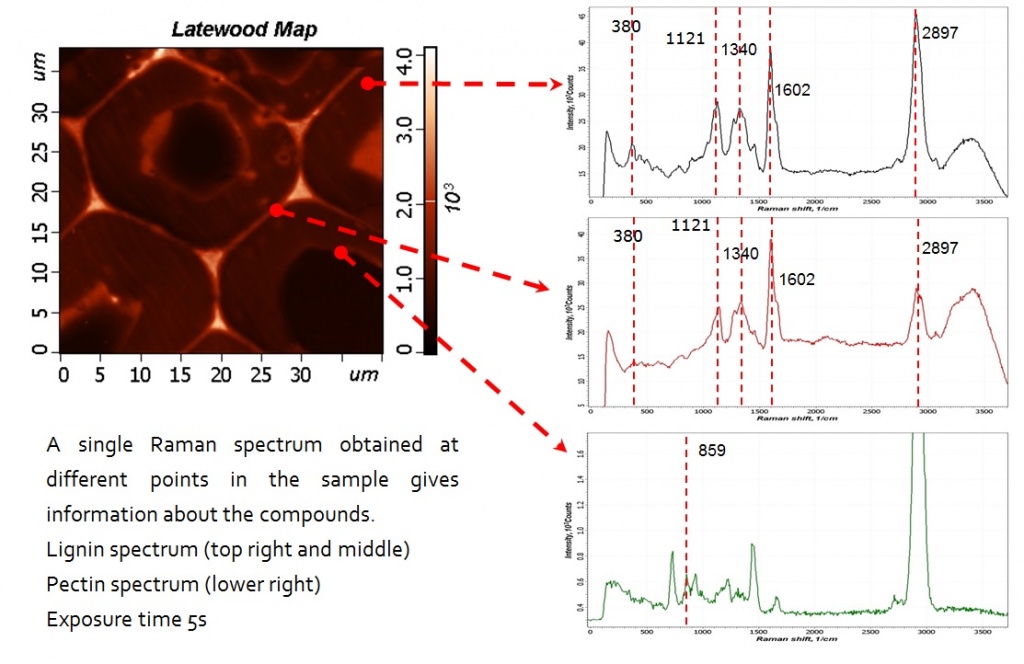
Plant cell wall imaging by confocal Raman microscopy
One of the first studies of Raman scattering in plant studies was carried out on flax stem tissue. Using the NIR-FT-Raman method, area maps were collected in 6–10 μm increments and chemical profiles were calculated by integrating over certain spectral regions. Using this method, no information was obtained at the cellular level, however, the distribution of the main components (cellulose, lignin, non-cellulose polysaccharides) was demonstrated for various types of anatomical tissues (epidermal tissue, scourer, fibers and the core). To investigate changes at the hierarchical level of individual cells and/or layers of the cell wall, a higher spatial resolution is necessary, and, as a result, the use of visible laser excitation is preferred. In recent years, the potential of Raman imaging has been demonstrated on the walls of wood cells. To study the cell walls of beech, a laser system of the near-IR range (785 nm) was used, and confocal Raman microscopes with a visible laser excitation wavelength (633 nm and 532 nm) were used to study the walls of wood cells with submicron spatial resolution.
|
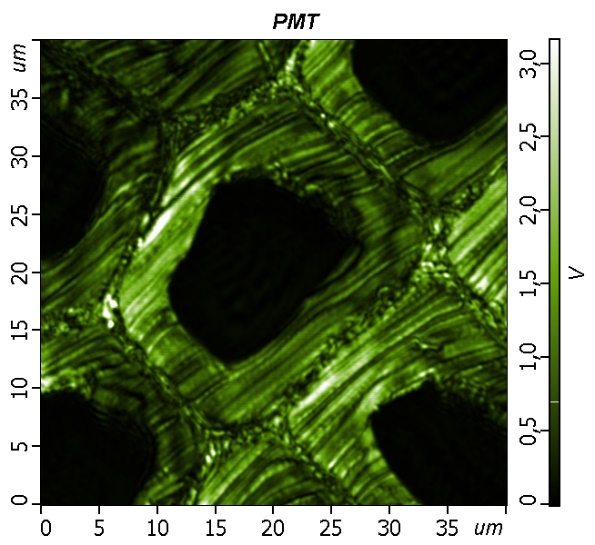
|
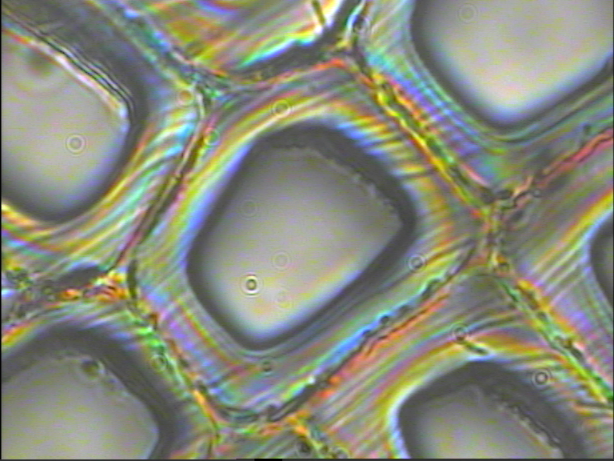
| The backscattered Rayleigh image of the area 40x40 µM obtained. The laser wavelength is 532 nm. The step size is about 80 nm. | The sample represents a cross-section of latewood cells. The optical image was obtained with Oil immersion 100x NA=1.3 objective. |
The potential of confocal Raman microscopy/imaging for non-destructive in situ studies of the walls of plant cells has already been demonstrated on various plant tissues. The high spatial resolution allows detecting changes in the polymer composition of the cell wall and/or orientation at the level of the cell and the cell wall. The Raman imaging approach allows obtaining spectra with a short integration time (0.2–2 s), which minimizes problems with sample degradation. The signal-to-noise ratio in the spectra is sufficient to visualize chemical and orientational changes that are not visible on the light optical micrograph. Extracting the average spectra with the best signal-to-noise ratio from the identified areas of interest can help in interpreting the image and reveal more details. Conclusions on all components of cells can be obtained in a non-destructive manner and in context with the structure. This main advantage and potential also have a disadvantage, since highly overlapping bands are sometimes difficult to interpret, and the conclusions on components with a small amount can be ambiguous.
However, additional experiments on other variants of the cell wall and selective chemical treatment, as well as combinations with other methods (eg, antibody probes) will improve our understanding of the generated Raman spectra in the future.